Topic How to find the k value: Finding the k value is essential for analyzing vapor-liquid equilibrium and identifying cluster patterns in data. By dividing the mole fraction in the vapor by the mole fraction in the liquid, the k value provides valuable insights into the composition and characteristics of a system. With this knowledge, users can gain a deeper understanding of their data and make informed decisions that contribute to better outcomes. Discovering the correct k value unlocks the potential to unlock hidden patterns and uncover meaningful information within a dataset, empowering users to make impactful discoveries.
Table of Content
- How to calculate the k-value for vapor-liquid equilibrium?
- What is the definition of the K value?
- How can you calculate the K value?
- YOUTUBE: Find the Value of K for which the Quadratic has Equal Roots: Quick and Simple Explanation
- What does the K value represent in vapor-liquid equilibrium?
- What is the formula for calculating the K value?
- How do you determine the mole fraction in the vapor and liquid?
- Can you provide an example of calculating the K value?
- Are there any specific conditions or assumptions for determining the K value?
- How is the K value used in the context of clustering data using K-Means?
- Are there any alternatives to finding the K value in certain scenarios?
How to calculate the k-value for vapor-liquid equilibrium?
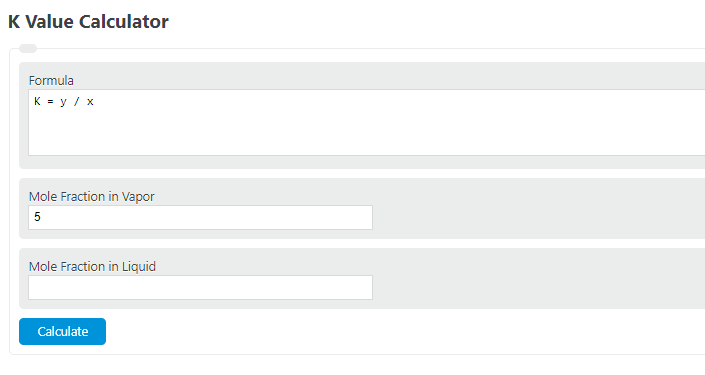
To calculate the K-value for vapor-liquid equilibrium, follow these steps:
1. Gather the necessary information: You will need the mole fraction in the vapor (yi) and the mole fraction in the liquid (xi).
2. Determine the mole fractions: The mole fraction can be calculated by dividing the moles of a particular component by the total moles in the mixture. You can use experimental data or molecular simulations to obtain these values.
3. Calculate the K-value: The K-value is calculated by dividing the mole fraction in the vapor by the mole fraction in the liquid. Mathematically, K = yi / xi.
4. Interpret the results: The K-value represents the equilibrium ratio between the mole fractions in the vapor and the liquid phases. It indicates how much of a certain component will exist in the vapor phase compared to the liquid phase at equilibrium.
Note: The K-value is specific to each component in the mixture and can vary depending on temperature and pressure. It is commonly used in processes such as distillation to understand the composition of the vapor and liquid phases.
READ MORE:
What is the definition of the K value?
The K value, also known as the equilibrium ratio, is a measure used in thermodynamics and chemical engineering to represent the ratio of the mole fraction of a substance in the vapor phase to its mole fraction in the liquid phase at a given temperature and pressure.
To calculate the K value, you divide the mole fraction of the substance in the vapor phase by its mole fraction in the liquid phase. The mole fraction is the ratio of the number of moles of the substance to the total number of moles present in the mixture.
The K value is frequently used in vapor-liquid equilibrium calculations to determine the composition of the vapor and liquid phases in equilibrium. It serves as a key parameter in various thermodynamic models and equations that describe the behavior of mixtures and phase equilibria.
In practical applications, the K value is often determined experimentally or estimated using thermodynamic models. These models take into account the nature of the substance, temperature, pressure, and the interactions between different components in the mixture. The accuracy of the K value calculation depends on the accuracy of the model used and the availability of experimental data.
Overall, the K value provides valuable information about the distribution of components between the vapor and liquid phases in a mixture and plays a significant role in understanding and predicting phase equilibria in various industrial processes.
How can you calculate the K value?
To calculate the K value, you need to know the mole fraction in both the vapor and the liquid. The K value represents the ratio of the mole fraction in the vapor to the mole fraction in the liquid.
Here are the steps to calculate the K value:
1. Determine the mole fraction in the vapor: The mole fraction is the ratio of the number of moles of a component to the total number of moles in the mixture. If you don\'t have the mole fraction directly, you may need to calculate it using other available data.
2. Determine the mole fraction in the liquid: Similarly, calculate the mole fraction in the liquid phase using the available data. Again, if you don\'t have the mole fraction directly, you may need to use other information to calculate it.
3. Divide the mole fraction in the vapor by the mole fraction in the liquid: This division gives you the K value. For example, if the mole fraction in the vapor is 0.3 and the mole fraction in the liquid is 0.1, then the K value would be 0.3/0.1 = 3.
Remember that the K value represents the equilibrium ratio between the vapor and the liquid phases in a system. It can be used to understand and predict the behavior of mixtures, particularly in vapor-liquid equilibrium calculations.
If you are specifically looking for the number of clusters in the context of K-Means clustering, that is a different concept. In K-Means clustering, the \"K\" refers to the predefined number of clusters into which you want to divide your data. Determining the optimal number of clusters is a separate topic and various methods like the elbow method or silhouette score can be used to find an appropriate value.
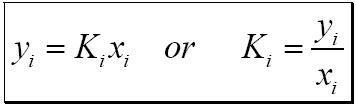
Find the Value of K for which the Quadratic has Equal Roots: Quick and Simple Explanation
Are you curious about the beauty and intricacy of quadratic equations? Unleash your mathematical prowess and dive into the world of quadratic functions in this captivating video. Discover the fascinating patterns and solutions that unfold as you explore the realm of parabolas and their unique properties. Get ready to be amazed by the elegance of quadratics!
Find the value k that makes the function continuous
Brace yourself for a journey through the realm of continuity in mathematics. In this mesmerizing video, witness the magic of unbroken flow and seamless connections. Delve into the concept of continuity and witness how functions smoothly transition from one point to another without any interruptions. Embark on this visual adventure and let the concept of continuity captivate your mind!
What does the K value represent in vapor-liquid equilibrium?
The K value represents the equilibrium ratio between the mole fractions of a component in the vapor phase and the liquid phase in a vapor-liquid equilibrium system. It is used to describe the extent of the distribution of a component between the two phases.
To understand the significance of the K value, let\'s consider a simple example. Imagine a closed system that contains a liquid and its corresponding vapor in equilibrium. If we have a component A present in both the liquid and vapor phases, the K value for component A would be calculated by dividing the mole fraction of A in the vapor phase (y_A) by the mole fraction of A in the liquid phase (x_A).
K value = y_A / x_A
The K value provides insights into how the component is distributed between the vapor and liquid phases. It represents the relative concentrations of the component in each phase. A K value greater than 1 indicates that the component is more concentrated in the vapor phase, while a K value less than 1 indicates a higher concentration in the liquid phase.
Furthermore, the K value can be used to predict the composition of the vapor and liquid phases at equilibrium. For example, if the K value for a component is known, along with the composition of one phase, the composition of the other phase can be calculated using the K value and the overall material balance.
In summary, the K value in vapor-liquid equilibrium represents the ratio of the mole fraction of a component in the vapor phase to the mole fraction of the same component in the liquid phase. It serves as an indicator of the relative concentrations of the component in each phase and can be used to predict the composition of the phases at equilibrium.
What is the formula for calculating the K value?
The formula for calculating the K value, also known as the vapor-liquid equilibrium ratio, is relatively straightforward. To calculate the K value, you need to divide the mole fraction in the vapor phase by the mole fraction in the liquid phase.
Specifically, the formula is:
K value = mole fraction in vapor / mole fraction in liquid
The mole fraction represents the ratio of moles of a particular component to the total moles of all components in a mixture. It is often denoted by the symbol \"x\" or \"y\" for liquid and vapor respectively.
To calculate the K value, you will need to know the mole fractions of the component in both the vapor phase and the liquid phase. Once you have these values, simply divide the mole fraction in the vapor by the mole fraction in the liquid to obtain the K value.
It\'s important to note that the K value is used in thermodynamics and can be applied in various contexts, such as in the analysis of vapor-liquid equilibrium or in the calculation of equilibrium constants for chemical reactions. The specific meanings and applications of the K value may vary depending on the context in which it is used.
_HOOK_
How do you determine the mole fraction in the vapor and liquid?
To determine the mole fraction in the vapor and liquid, you can follow these steps:
1. Understand the concept:
- The mole fraction represents the ratio of moles of a specific component to the total moles in a mixture.
- The vapor phase is the gaseous state of a substance, while the liquid phase is the liquid state.
- The mole fraction in the vapor is the ratio of moles of the component in the vapor phase to the total moles in the vapor phase.
- The mole fraction in the liquid is the ratio of moles of the component in the liquid phase to the total moles in the liquid phase.
2. Gather the necessary information:
- You need to know the total moles of the component in the mixture.
- You need to measure or determine the number of moles of the component in the vapor and liquid separately.
3. Calculate the mole fraction:
- To find the mole fraction in the vapor, divide the number of moles of the component in the vapor phase by the total moles in the vapor phase.
- To find the mole fraction in the liquid, divide the number of moles of the component in the liquid phase by the total moles in the liquid phase.
4. Example calculation:
Let\'s say you have a mixture of water and ethanol, and you want to determine the mole fraction of ethanol in the vapor and liquid phases.
- Assume you have determined that there are 5 moles of ethanol in the vapor phase, and the total moles in the vapor phase are 10.
- Calculate the mole fraction in the vapor: 5 moles ethanol / 10 total moles = 0.5 mole fraction of ethanol in the vapor.
- Assume you have determined that there are 20 moles of ethanol in the liquid phase, and the total moles in the liquid phase are 100.
- Calculate the mole fraction in the liquid: 20 moles ethanol / 100 total moles = 0.2 mole fraction of ethanol in the liquid.
5. Interpreting the results:
- The mole fractions represent the relative amounts of the component in the vapor and liquid phases.
- In the example above, ethanol has a higher mole fraction in the vapor phase (0.5) compared to the liquid phase (0.2), indicating that ethanol is more volatile and tends to evaporate more readily.
Remember, the specific method to determine the moles in vapor and liquid may vary depending on the specific situation or experimental setup.
Can you provide an example of calculating the K value?
Sure! I can provide an example of calculating the K value.
The K value, also known as the vapor-liquid equilibrium ratio, represents the ratio of the mole fraction of a component in the vapor phase to the mole fraction of the same component in the liquid phase.
To calculate the K value, you would need to know the mole fractions of the component in both the vapor and liquid phases. Let\'s say we have a component A in a two-phase system and we know its mole fraction in the vapor phase (yi) is 0.8 and in the liquid phase (xi) is 0.4.
The formula to calculate the K value is:
K = yi / xi
Plugging in the values:
K = 0.8 / 0.4
Evaluating the expression, we get:
K = 2
So, the K value for the component A in this example is 2.
This means that for every mole of component A in the liquid phase, there are 2 moles of component A in the vapor phase.
Remember, this is just a simple example and the calculation of K value can vary depending on the specific system and conditions.

Are there any specific conditions or assumptions for determining the K value?
Yes, there are specific conditions and assumptions for determining the K value in different contexts. Since you\'ve mentioned finding the k value, it seems like you might be referring to the context of vapor-liquid equilibrium (VLE).
In VLE, the K value is a measure of the distribution or partitioning of a component between the vapor and liquid phases of a mixture. The K value represents the ratio of the mole fraction of a component in the vapor phase (yi) to the mole fraction of the same component in the liquid phase (xi).
To determine the K value in VLE, you typically need experimental data or reliable thermodynamic models. The conditions and assumptions for determining the K value depend on the specific system and the available information. Here is a general step-by-step process to determine the K value:
1. Collect data or select a thermodynamic model: You need to have either experimental data or a thermodynamic model that can describe the behavior of the system you are studying. Reliable models like the Peng-Robinson equation of state or the Soave-Redlich-Kwong equation of state are commonly used.
2. Define the system: Specify the components present in the system and their respective mole fractions in the liquid and vapor phases. The system can consist of one or more components.
3. Determine the equilibrium condition: In VLE, the vapor and liquid phases are in equilibrium with each other. This means that the chemical potential of each component in the vapor and liquid phases should be equal. You can express this equilibrium condition using a mathematical expression or a set of equations, often called equilibrium equations.
4. Solve the equilibrium equations: Use the selected thermodynamic model or experimental data to solve the equilibrium equations. This can involve iterative calculations to converge on a solution.
5. Compute the K value: Once you have obtained the solutions for the equilibrium equations, you can determine the K value for each component by dividing the mole fraction in the vapor phase by the mole fraction in the liquid phase (K = yi / xi).
It\'s important to note that the specific conditions and assumptions can vary depending on the system you are studying. These conditions might include temperature, pressure, and the presence of any other components or phases in the system. Additionally, different models may have different assumptions and limitations, so it\'s essential to choose an appropriate model for your specific application.
Overall, determining the K value involves a combination of experimental data, thermodynamic models, and mathematical calculations to describe the behavior of a system in vapor-liquid equilibrium.
How to Calculate Equilibrium Constant K Value Practice Problems and Examples: Step by Step Explanation
Explore the concept of equilibrium constant and unravel the mysteries of chemical reactions in this thought-provoking video. Join us as we delve into the realm of dynamic equilibrium, where the forces of forward and reverse reactions balance perfectly. Witness the fascinating concept of chemical equilibrium come to life, as we navigate through the principles and applications of the equilibrium constant. Prepare to be amazed by the delicate balance that governs chemical systems!
Arithmetic Sequence: Solving for the Value of K
Join us on a journey through the captivating world of arithmetic sequences. In this illuminating video, witness the mesmerizing patterns and symmetries that arise from adding the same fixed number repeatedly. Uncover the secrets of arithmetic sequences and witness how they can be used to unlock a multitude of mathematical puzzles and problems. Prepare to be enthralled by the precision and fascinating properties of arithmetic sequences!
How is the K value used in the context of clustering data using K-Means?
In the context of clustering data using K-Means, the K value refers to the number of clusters that you want to form. It determines how many groups or clusters the algorithm should create to partition the data.
To find the optimal K value, you can use various methods such as the Elbow Method or the Silhouette Method. Here\'s a step-by-step approach:
1. Start by plotting the sum of squared distances (or SSE) against different values of K. The SSE measures the distance between each data point and the centroid of its assigned cluster.
2. Run the K-Means algorithm for different values of K. In each iteration, calculate the SSE and store it.
3. Plot the SSE values against the corresponding K values.
4. Look at the resulting plot and identify the \"elbow\" point, which is the point of inflection where the SSE starts to decrease at a slower rate. This elbow point represents the optimal K value.
5. Additionally, you can use the Silhouette Method, which considers both the cohesion (distance within the cluster) and separation (distance between clusters). Calculate the average silhouette score for each K value and choose the one with the highest score.
It\'s important to note that the optimal K value may not always be clear-cut, and it often requires subjective interpretation. You may need to consider domain knowledge or the specific goals of your analysis.
Once you have determined the appropriate K value, you can use it in the K-Means algorithm to cluster your data into the desired number of groups. The algorithm iteratively assigns data points to the nearest cluster centroid and updates the centroids until convergence, resulting in the final cluster assignments.

READ MORE:
Are there any alternatives to finding the K value in certain scenarios?
Yes, there are alternative methods to finding the K value in certain scenarios. The specific alternative method would depend on the context or application in which you are trying to find the K value. Here are a few potential alternatives:
1. Experimental Methods: In some cases, it may not be possible to calculate the K value directly using equations or models. In such scenarios, experimental methods can be used to determine the K value. This involves conducting experiments or measurements to determine the equilibrium concentrations or properties of the components involved.
2. Reference Tables and Databases: In certain fields, there may be reference tables or databases available that provide K values for common substances or systems. These tables or databases are often based on experimental data or theoretical calculations and can be used to find approximate K values for specific scenarios.
3. Thermodynamic Models: Thermodynamic models, such as the Peng-Robinson or Soave-Redlich-Kwong equations of state, can be used to estimate K values for certain systems. These models use mathematical equations and parameters to predict the behavior of components in equilibrium and can be particularly useful when experimental data is unavailable or limited.
4. Computational Simulations: Another alternative is to use computational simulations, such as molecular dynamics or Monte Carlo methods, to predict K values. These simulations involve modeling the molecular interactions and movements of the components in the system to estimate equilibrium properties, including K values. However, these methods can be computationally intensive and may require specialized software or expertise.
It is important to note that the choice of alternative method for finding the K value will depend on the specific scenario, available data, and level of accuracy required. It is recommended to consult relevant literature, domain experts, or utilize specific software or tools for the particular application to determine the most suitable alternative method.
_HOOK_